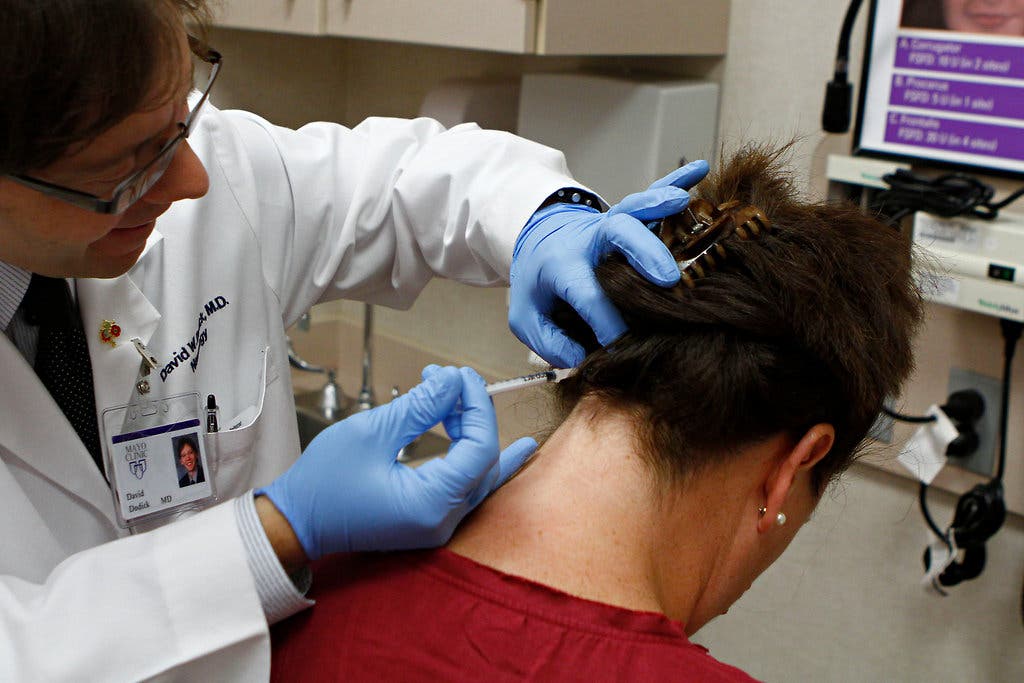
The FDA (Food and Drug Administration) announced on Friday that they have given Allergan Inc. the approval to sell Botulinum toxin as a treatment to adult patients suffering from chronic migraines.
People who suffer from headaches for more that 15 days on a monthly basis will now be able to benefit from the wonders of botox.
The product which is known for its aesthetic usage against wrinkles will be injected into the individuals’ neck or head every 12 weeks by a medical professional in order to eliminated future head pain.
Russell Katz, director of the FDA’s division of neurology products, said in the statement:
“Chronic migraine is one of the most disabling forms of headache.This condition can greatly affect family, work, and social life, so it is important to have a variety of effective treatment options available.”
Back in the 1990s, doctors noticed that many of their patients who originally came to see them for a touch up on their little lines and wrinkles were reporting that something else was happening whenever hey were injected with the protein which is produced by the bacterium Clostridium botulinum- their migraines would vanish.
So after many years of research – physicians were able to have conclusive results to present to the American Headache Society which is centered in Chicago which eventually fought and convinced the administration to accepted the fact that the product was also a medicine.
Allergan which won the rights back in July to sell Botox as a migraine treatment in England made $1.3 billion selling the well known neurological product last year and according to experts they will make an extra billion in 2012.
The Irvine, California-based pharmaceutical company is set to conquer the rest of the European markets as soon as possible.