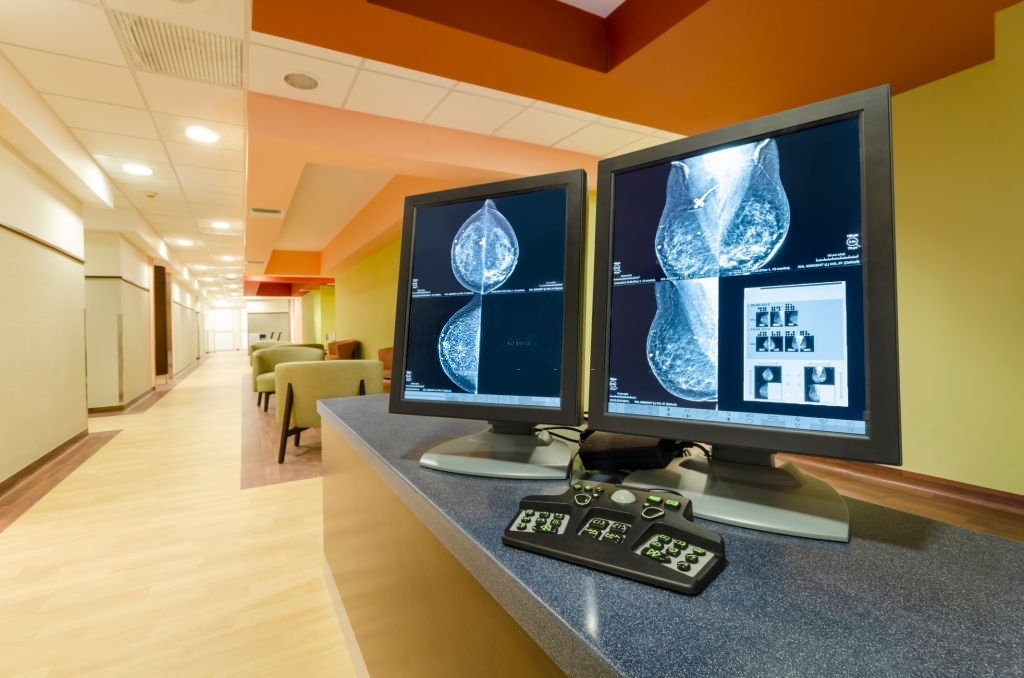
The Food and Drug Administration, FDA, has approved for the first time, a 3D mammography imaging device that is hoped will aid doctors in detecting more cancerous breast tumors.
The system is called the Selenia Dimensions System produced by the Hologic Inc. company and the system provides both 2D and 3D X Ray images thus giving doctors additional viewpoints from where to make diagnosis.
Although the system doubles the radiation doses to patients, the FDA notes that it can also increase the accuracy of diagnosis thus limiting the number of examinations overall.
The FDA’s Device Division director Jeffrey Shuren pointed out the benefits of the device in that it could enhance diagnosis and treatment approaches.
It was two studies that the FDA based their approval on as the tests showed a seven percent improvement in detecting cancerous tumors when viewing the images from the device compared to the techniques using traditional 2D technologies.
The 3D approach allows doctors to examine tissue that might normally be obscured by overlapping tissue.
Since one in eight women will be diagnosed with breast cancer over a lifetime approximately, the National Cancer Institute recommends that women between the ages of 40 on up should have a mammogram every one to two years.
The Selenia device has already seen acceptance in Europe, South America, and in Asia.
The Bedford, Massachusetts company Hologic had their shares rise by 36 cents to $20.09 in afternoon stock trading.